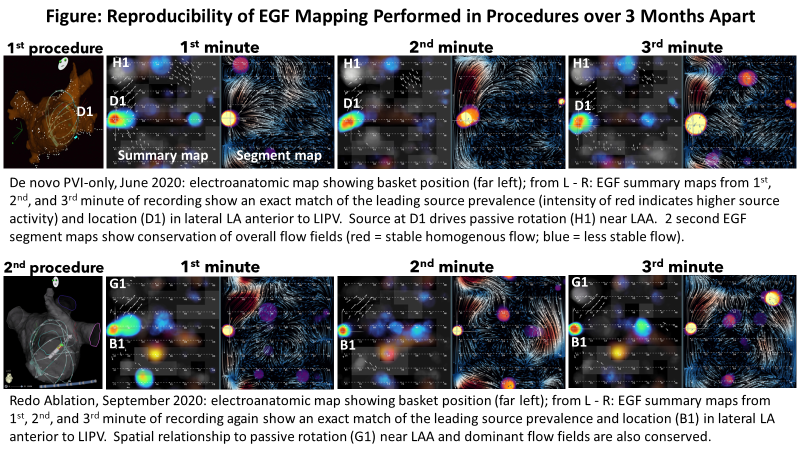
Inter-Procedural Reproducibility of EGF Mapping
Between Procedures Separated by 3 Months or Longer
Petr Neuzil, MD, PhD; Melissa H. Kong, MD; Peter Ruppersberg, MD; Moritoshi Funasako, MD, PhD; Jan Petru, MD; Vivek Reddy, MD
Background
Over the last decade, various algorithms for localizing extra-pulmonary vein (PV) AF drivers/sources (focal or rotational activity) have been studied. Validating these mapping systems is confounded by lack of ground truth, typically manifesting as poor map reproducibility. Short of optical mapping studies, it remains difficult to prove that identified drivers are true drivers, so other criteria are needed to buttress the specificity of identified sources. Electrographic flow (EGF) mapping uses a novel technique to create full, near real-time spatiotemporal visualizations of atrial electrical wavefront propagation to identify putative atrial fibrillation (AF) sources.
Objectives
To evaluate whether AF source activity identified by EGF mapping is reproducible over procedures separated by ≥ 3 months.
Methods
As part of the FLOW-AF trial (NCT04473963), 18 pts underwent de novo balloon-based PVI and post-PVI EGF mapping with a 64-pole basket catheter. At ~3 months, a remap procedure included PVI check + EGF mapping. Confirmation with ≥20 min wait to confirm isolation was required for post-PVI EGF mapping in both procedures. One pt was excluded as the post-PVI basket position was not stored with the EGF map such that anatomic location could not be verified, and 5 pts were excluded since AF was non-inducible at remapping.
Results
Of the 12 pts who were fully mapped and re-mapped (at 99.7 ± 20.9 days post-PVI), after de novo PVI, 18 sources with activity > 26% (the threshold to qualify a source as legitimate) were detected (10 RA, 8 LA, 7 both atria). The remap procedures revealed that 16 of 18 (89.0%) sources were at the same anatomic location as in the initial procedure; the remaining 2 sources were detected in the SVC after the initial procedure post-PVI and were again seen in the SVC on remap, but in a different aspect of the SVC. One patient did not have any sources detected post-PVI in the de novo procedure, but was found to have an RA source after the PVs were re-isolated during the remap procedure.
Conclusions
EGF mapping produces reproducible results from one procedure to the next, such that if a clinically relevant source was detected post-PVI during the first procedure, but was not ablated, the source was again detectable at remapping performed at 3-mo after de novo PVI. This inter-procedural reproducibility may enable longitudinal follow-up of individual patients’ AF pathophysiology.
