Vivek Y. Reddy, MD; Steven Castellano, PhD; Tamas Szili-Torok, MD, PhD; Jan Petru, MD; Moritoshi Funakawa, MD, PhD; Mark Hoogendijk, MD, PhD; Sip Wijchers, MD; Stefan G. Spitzer, MD; Andreas Rillig, MD; Petr Neuzil, MD, PhD; Melissa H. Kong, MD
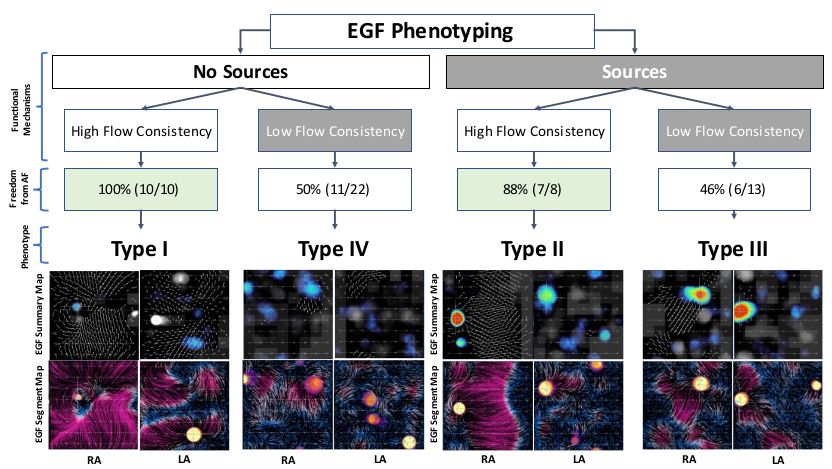
phenotypes exist based on the presence or absence of active EGF-identified sources and whether
electrographic flow consistency (EGFC) is high v. low. Type I patients have no sources and high EGFC and
in the FLOW-AF trial were treated with PVI-only with 100% freedom from AF (FFAF) at 1 year post-
ablation. Type II patients had EGF-identified sources and high EGFC and underwent PVI + targeted EGF
source ablation with 88% FFAF. Type III patients also had EGF-identified sources but low EGFC and
despite PVI + targeted EGF source ablation had only 46% FFAF. Similarly, Type IV patients who did not
have sources but had low EGFC and PVI-only had only 50% FFAF. The ability to identify which individual
patients will benefit from PVI-only v. PVI + source ablation will enable the physician to tailor ablation
strategies to optimize outcome.
Background
The randomized controlled FLOW-AF trial showed that the presence of a clinically relevant EGF-identified source left unablated results in worse outcomes post-ablation. Electrographic Flow (EGF) mapping not only identifies these active sources of excitation, but can also estimate the consistency of observed atrial wavefront propagation. Electrographic flow consistency (EGFC) is computed from the Euclidean length of vector field estimates over time and may provide additional insight into an individual patient’s atrial fibrillation (AF) disease that in combination with EGF-identified sources may enable the phenotyping of persistent AF (PeAF) patients.
Objectives
1) Determine relationship between EGFC and AF recurrence.
2) Propose a phenotyping framework for treatment and prognosis based on functional mechanisms as described by EGF.
Methods
In FLOW-AF, patients prospectively underwent PVI and subsequent EGF-guided source ablation in all cases when such sources were identified, PVI-only when no sources were seen. In each patient, a series of 1-minute EGF recordings were taken in multiple locations per atrium post-PVI but pre- any adjunctive or source ablation. Mean EGFC from these recordings were averaged for each basket position and these averages were averaged to determine an overall EGFC across all locations. A decision tree classifier was
employed to determine the optimal splitting point below which EGFC scores were more commonly associated with recurrence within 12 months.
Results
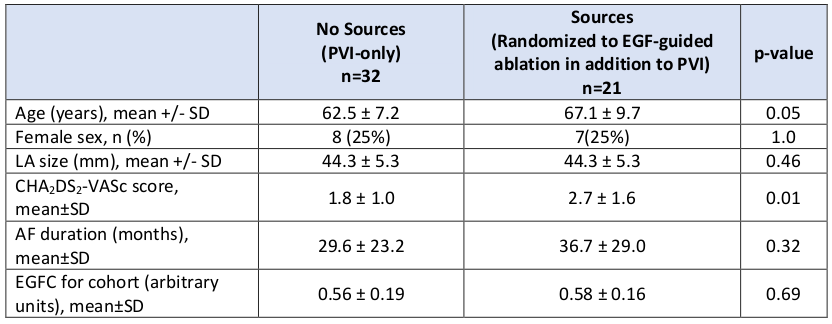
Based on FLOW-AF trial design, patients were divided into those with no sources (n=32) v. those with sources above threshold randomized to EGF-guided ablation (n=21). Baseline characteristics in Table. Decision tree classifier identified 0.63 as EGFC above which recurrence is least common and below which recurrence is most common. Patients with high EGFC and no sources had 100% (10/10) freedom from AF (FFAF); patients with high EGFC and at least 1 ablated source had 88% (7/8) FFAF; patients with low EGFC and no sources had 50% (11/22) FFAF; and patients with low EGFC and at least 1
ablated source had 46% (6/13) FFAF.
Conclusions
Using EGF mapping algorithms to detect the presence of functional mechanisms of AF, the clinically heterogeneous population of PeAF patients can be stratified into distinct EGF phenotypes that guide the minimum-required ablation strategy and also provides post-ablation prognosis. A larger prognostication study is warranted to assess the clinical utility of this framework.
